

Let take percentage abundance of $^$, therefore we take the average of all chlorine atoms.
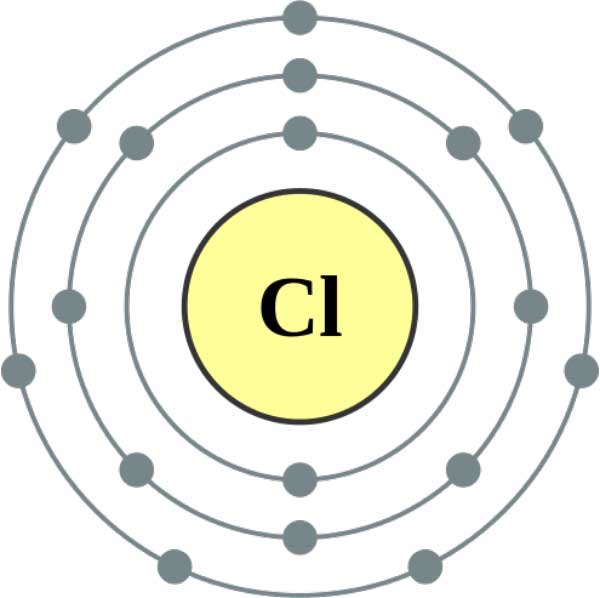
From the above calculation we can say that 39 gram of metal reacts with 35.5 g of chlorine. Relative atomic mass of chlorine is as follows: Relative atomic mass 75 100 × 35 + 25 100 × 37 35. The average mass of an elements naturally occurring isotopes compared to the mass of an atom of 12 C is defined as its relative atomic mass. We know that isotopes are defined as the species that are one of two or more species of atoms of an element which have the same atomic number and position in the periodic table but different atomic masses. Thus mass of metal mass of metal chloride - mass of chlorine (74.5-35.5) 39 g Here, we know that equivalent weight is the weight of a substance which reacts with 35.5 gram of chlorine. Due to the existence of isotopes, the atomic mass of chlorine is 35. Chlorine has two common isotopes, Chlorine-35 and Chlorine-37. Isotopes are atoms with the same number of protons (in the case of chlorine that means 17 protons) but different numbers of neutrons. Let suppose any atom has two isotopes then the formula of average relative atomic mass of atom is Average relative atomic mass of a atom= /$100$. The answer to why chlorine has an atomic weight of 35.5 not 35 is because of something called isotopes. Hint : We will solve this problem with the help of the formula of finding the average relative atomic mass of an atom when atomic masses of their isotopes are known.


 0 kommentar(er)
0 kommentar(er)
